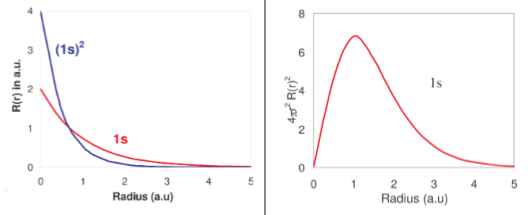
Orbitals
Quantum Numbers
Principal quantum number
- 1, 2, 3…
- Electron shell, electron energy and size of orbital Orbital Angualar Momentum Number
- Shape of the orbital
- 0 = s
- 1 = p
- 2 = d Z-component / Magentic of
- to
- Orientation of orbital
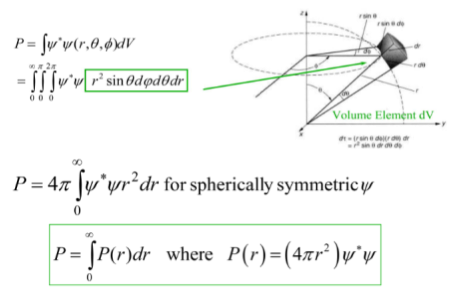
Filling
- Aufbau
- Start lowest in energy
- Pauli’s Exclusion
- Max two electrons per orbital
- No two electrons can have same n, l, m, s tuple
- Hund’s Rule of Maximum Multiplicity
- Orbitals with same energy filled one at a time
- Degenerate
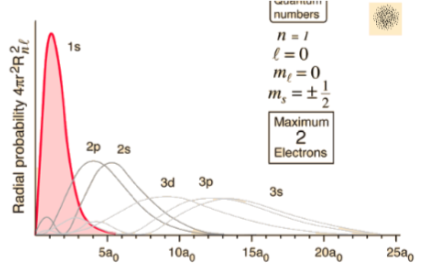
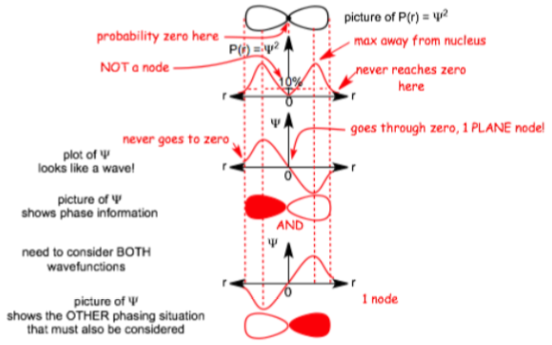
Radial
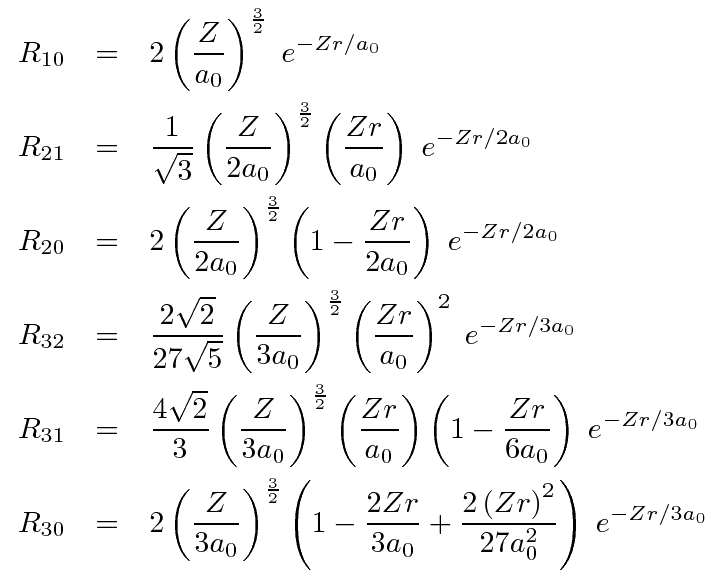
- Z = Atomic number
- Bohr radius
- Normalisation